![SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+ SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+](https://cdn.numerade.com/ask_previews/c07def3e-6d56-40ff-a5e3-203dd65cb17f_large.jpg)
SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+
Theoretical Study of Oxovanadium(IV) Complexation with Formamidoximate: Implications for the Design of Uranyl-Selective Adsorben
A Coordination Chemistry Study of Hydrated and Solvated Cationic Vanadium Ions in Oxidation States +III, +IV, and +V in Solution
![Cover Picture: [Fe(H2O)5(NO)]2+, the “Brown‐Ring” Chromophore (Angew. Chem. Int. Ed. 25/2019) - Monsch - 2019 - Angewandte Chemie International Edition - Wiley Online Library Cover Picture: [Fe(H2O)5(NO)]2+, the “Brown‐Ring” Chromophore (Angew. Chem. Int. Ed. 25/2019) - Monsch - 2019 - Angewandte Chemie International Edition - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/994a8c38-95fb-4e9e-a4c6-43434d2ce00c/anie201905910-toc-0001-m.jpg)
Cover Picture: [Fe(H2O)5(NO)]2+, the “Brown‐Ring” Chromophore (Angew. Chem. Int. Ed. 25/2019) - Monsch - 2019 - Angewandte Chemie International Edition - Wiley Online Library
![Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram](https://www.researchgate.net/publication/286004505/figure/fig6/AS:1132193107722252@1646947280620/Orientations-of-the-VO-ions-and-VOH2O5-complexes-in-the-ZAPH-lattice-a.jpg)
Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram

Transition Metals Occupy the d-block of periodic table Have d-electrons in valence shell Some characteristics of Transition Metals and their compounds. - ppt download
![Vanadium transition metal Chemistry ions vanadium(II) V2+ vanadium(III) V3+ vanadium(IV) [VO2]2+ vanadium(V) VO2+ vanadate VO43- redox chemical reactions complexes demonstration of oxidation states principal oxidation states +2 +3 +4 +5 GCE AS Vanadium transition metal Chemistry ions vanadium(II) V2+ vanadium(III) V3+ vanadium(IV) [VO2]2+ vanadium(V) VO2+ vanadate VO43- redox chemical reactions complexes demonstration of oxidation states principal oxidation states +2 +3 +4 +5 GCE AS](https://www.docbrown.info/page07/SSquestions/0complexVH2O6b.gif)
Vanadium transition metal Chemistry ions vanadium(II) V2+ vanadium(III) V3+ vanadium(IV) [VO2]2+ vanadium(V) VO2+ vanadate VO43- redox chemical reactions complexes demonstration of oxidation states principal oxidation states +2 +3 +4 +5 GCE AS

A Coordination Chemistry Study of Hydrated and Solvated Cationic Vanadium Ions in Oxidation States +III, +IV, and +V in Solution and Solid State | Inorganic Chemistry
Theoretical Study of Oxovanadium(IV) Complexation with Formamidoximate: Implications for the Design of Uranyl-Selective Adsorben
![SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+ SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+](https://cdn.numerade.com/ask_previews/c07def3e-6d56-40ff-a5e3-203dd65cb17f.gif)
SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+
![Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram](https://www.researchgate.net/publication/286004505/figure/fig2/AS:1132193107722244@1646947280476/EPR-spectrum-of-a-VO-doped-ZAPH-single-crystal-with-the-magnetic-field-at-0-from-the_Q320.jpg)
Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram
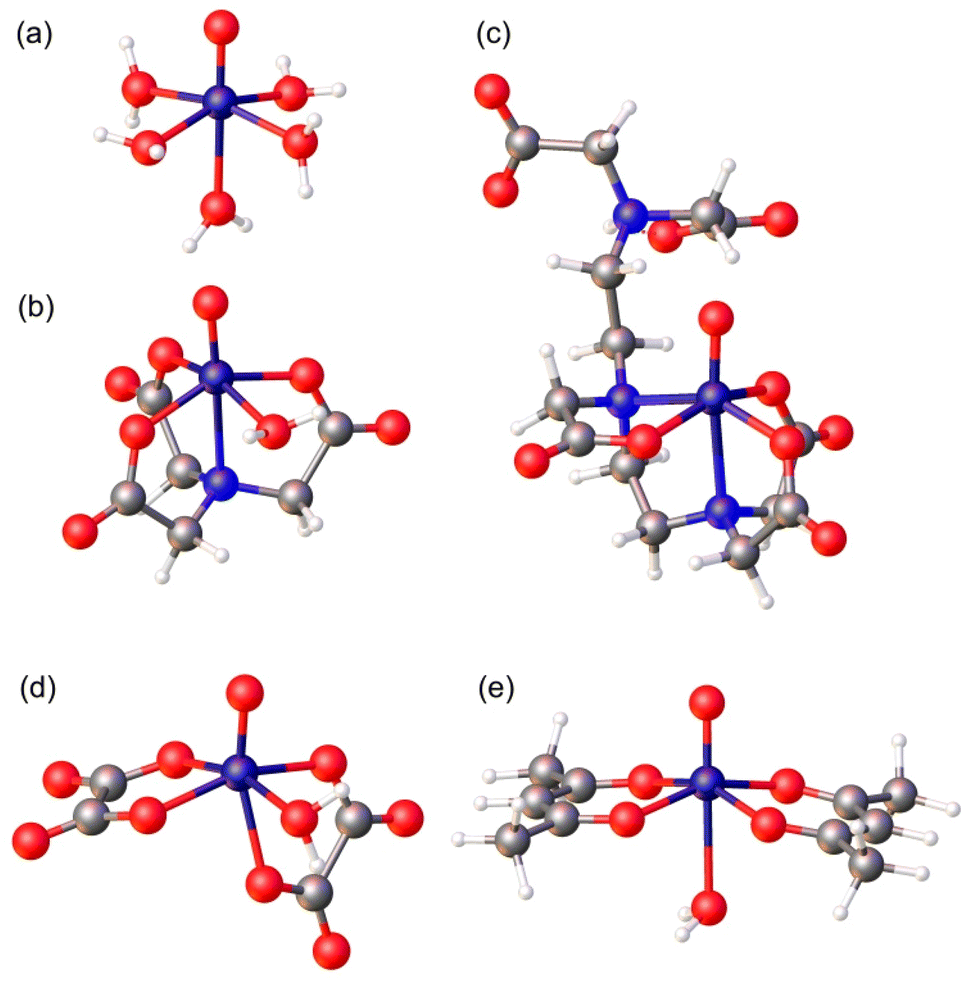
Magnetic and relaxation properties of vanadium( iv ) complexes: an integrated 1 H relaxometric, EPR and computational study - Inorganic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D2QI02635J

Control of monomeric Vo's versus Vo clusters in ZrO2−x for solar-light H2 production from H2O at high-yield (millimoles gr−1 h−1) | Scientific Reports
A Coordination Chemistry Study of Hydrated and Solvated Cationic Vanadium Ions in Oxidation States +III, +IV, and +V in Solution

Effect of the solvent on the formation of new oxovanadium(IV) complexes with pentafluorobenzoate anions and 1,10-phenanthroline - ScienceDirect
![SOLVED: Text: Question 3 A - Oxidation states Vanadium Species VO2+ V3+ Oxidation State Colour Complex Ion [VO2(H2O)4]+ VO(H2O)5]2+ [V(H2O)6]3+ B - Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)4]+ [VO(H2O)5]2- [ SOLVED: Text: Question 3 A - Oxidation states Vanadium Species VO2+ V3+ Oxidation State Colour Complex Ion [VO2(H2O)4]+ VO(H2O)5]2+ [V(H2O)6]3+ B - Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)4]+ [VO(H2O)5]2- [](https://cdn.numerade.com/ask_images/68c789418d3e4f1aabcb5b353a4e4c33.jpg)