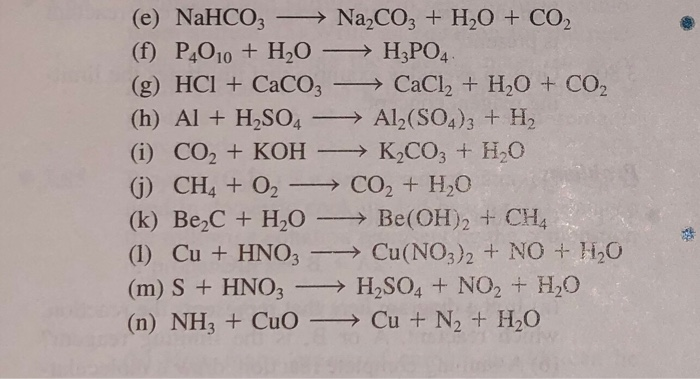
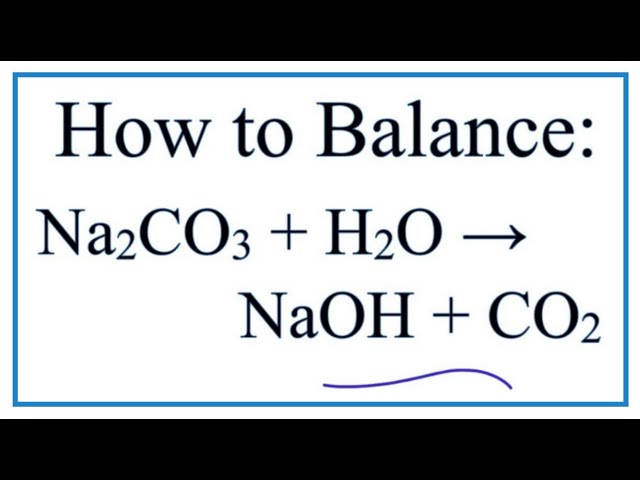
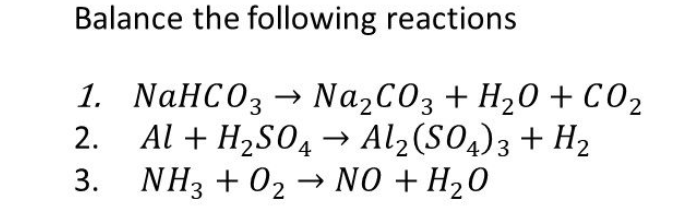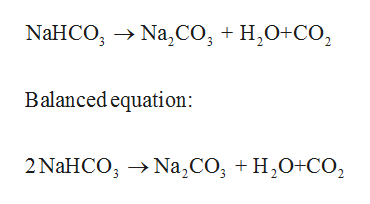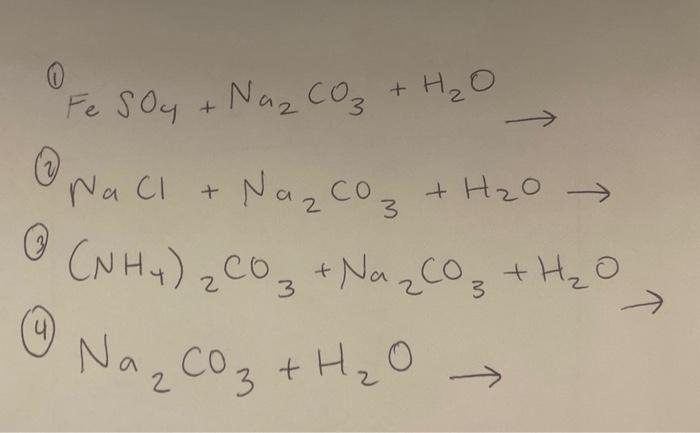How to Balance Na2CO3+H2O→NaOH+CO2 | How to Balance Na2CO3+H2O→NaOH+CO2 | By Chemistry 360 | Facebook
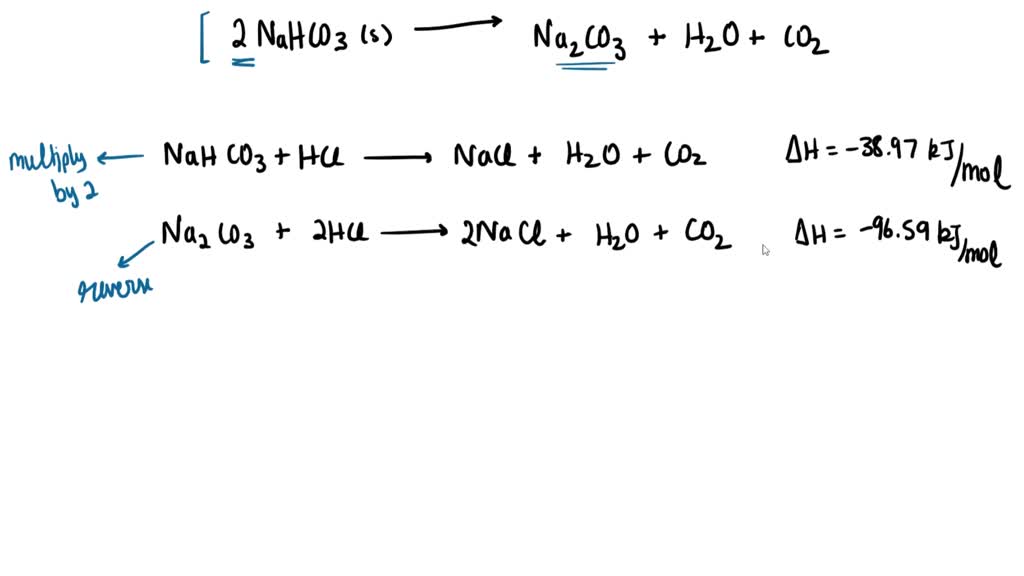
SOLVED: The following data are needed for this question: NaHCO3 (s) + HCl (aq) â†' NaCl (aq) + H2O (l) + CO2 (g) ΔH = -38.97 kJ mol-1 Na2CO3 (s) + 2HCl (

The Facile Hydrolysis of Imidazolinium Chlorides (N‐Heterocyclic Carbene Precursors) Under Basic Aqueous Conditions - Touj - Chemistry – A European Journal - Wiley Online Library

NaHCO3 decomposes as : 2NaHCO3(s) Na2CO3(s) + CO2(g) + H2O(g) The equilibrium pressure is 1.04 atm. The Kp the reaction is : (1) 0.2704 (2) 2.704 (3) 27.04 (4) 270.4

In the following sequence of reactions. Identify (E): NMO2 Na2CO3 + H2O + Co,- (4) AZach, A (C) + (D) 1 - NaOH (E) (a) NaHCO (b) Na2O2 (a) NaZnO2 (d) ZnCo nosition temneratures of alkaline earth