
H2+O2=H2O balance the chemical equation @mydocumentary838. h2+o2=h2o balance the chemical equation. - YouTube

31) The equilibrium constant the reaction H2O + CO) = H2(g) + CO2(g) is 0.44 1260K. The equilibrium constant the reaction 2H2(g) + 2C029) 7=2C0g + 2H2O(g) 1260 K is equal to

Reaction of CO, H2O, H2 and CO2 on the clean as well as O, OH and H precovered Fe(100) and Fe(111) surfaces - Catalysis Science & Technology (RSC Publishing)

calculate the equilibrium constant of H2 + O2 gives us H2O + CEO at 13957 if the equilibrium constant 135 - Chemistry - Equilibrium - 13886927 | Meritnation.com

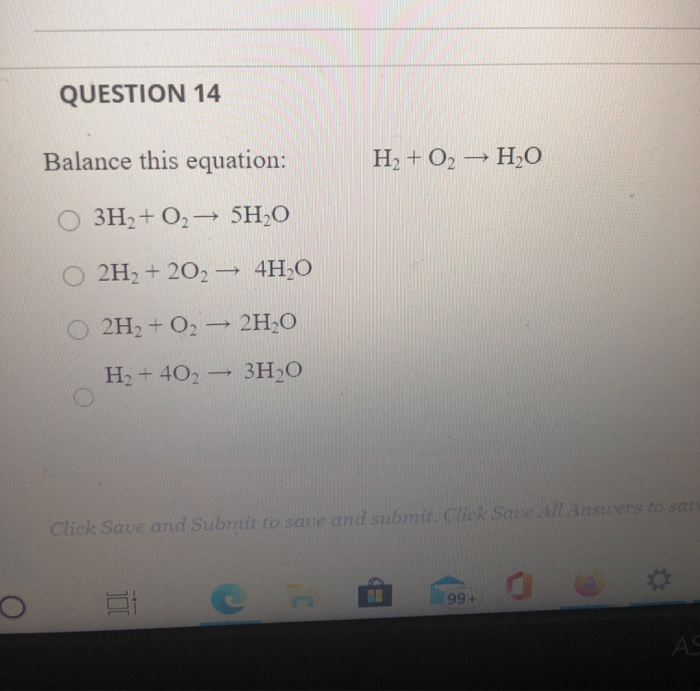
hydrogen reacts with oxygen to form water (H2O) according to the following equation: 2H2 + O2 → 2H2O what - brainly.com
3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting reagent ii)Max amount of H2O formed iii) Amount of reactants which remains unreacted.

PVT properties and diffusion characteristics of H2O/H2/CO2 mixtures in graphite nanoslits - ScienceDirect
Kp for the reaction CO2 + H2 =CO + H2O is found to be 16 at a given temperature. Originally equal number of moles of H2 and CO2 were placed in the
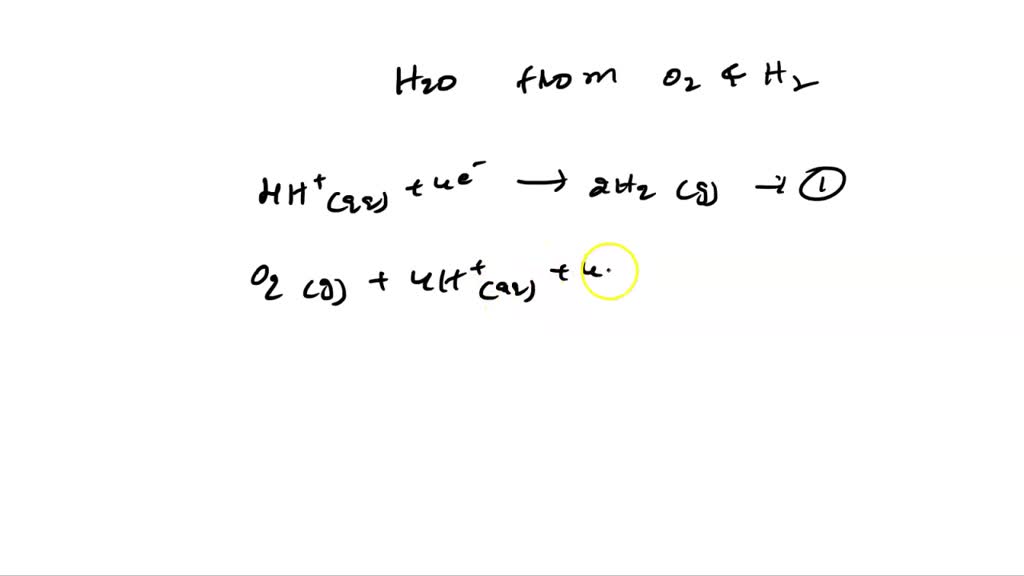


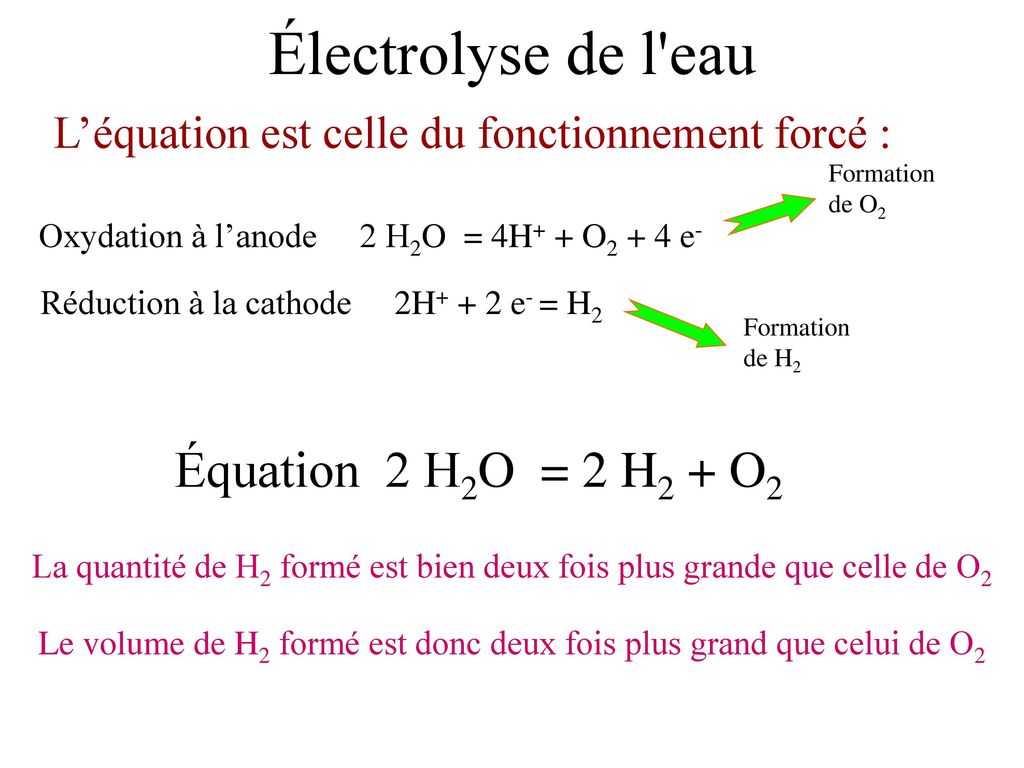


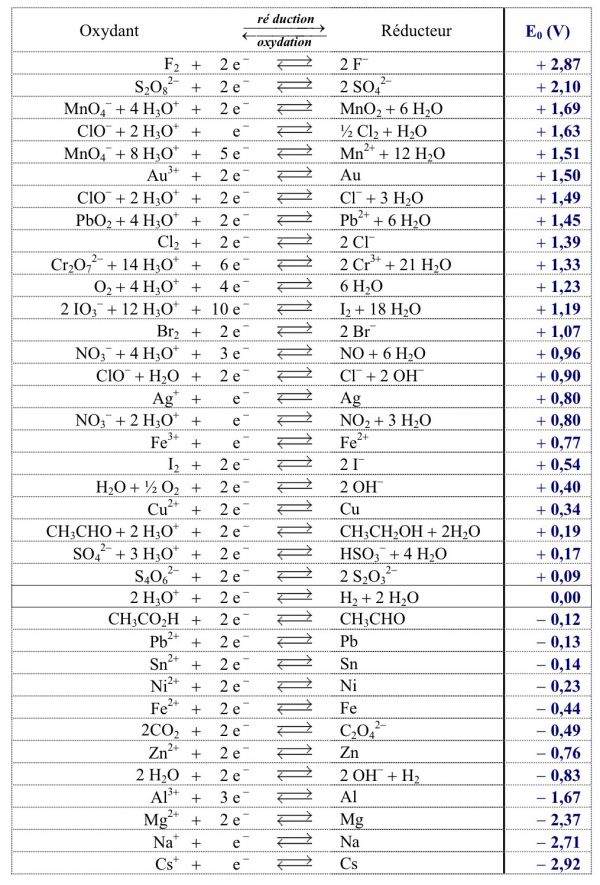



![PDF] Two triple points in the H2O–H2 system† | Semantic Scholar PDF] Two triple points in the H2O–H2 system† | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0e3a3f978107aae0e7b1ab47beca2a2ce22bd231/2-Figure1-1.png)



