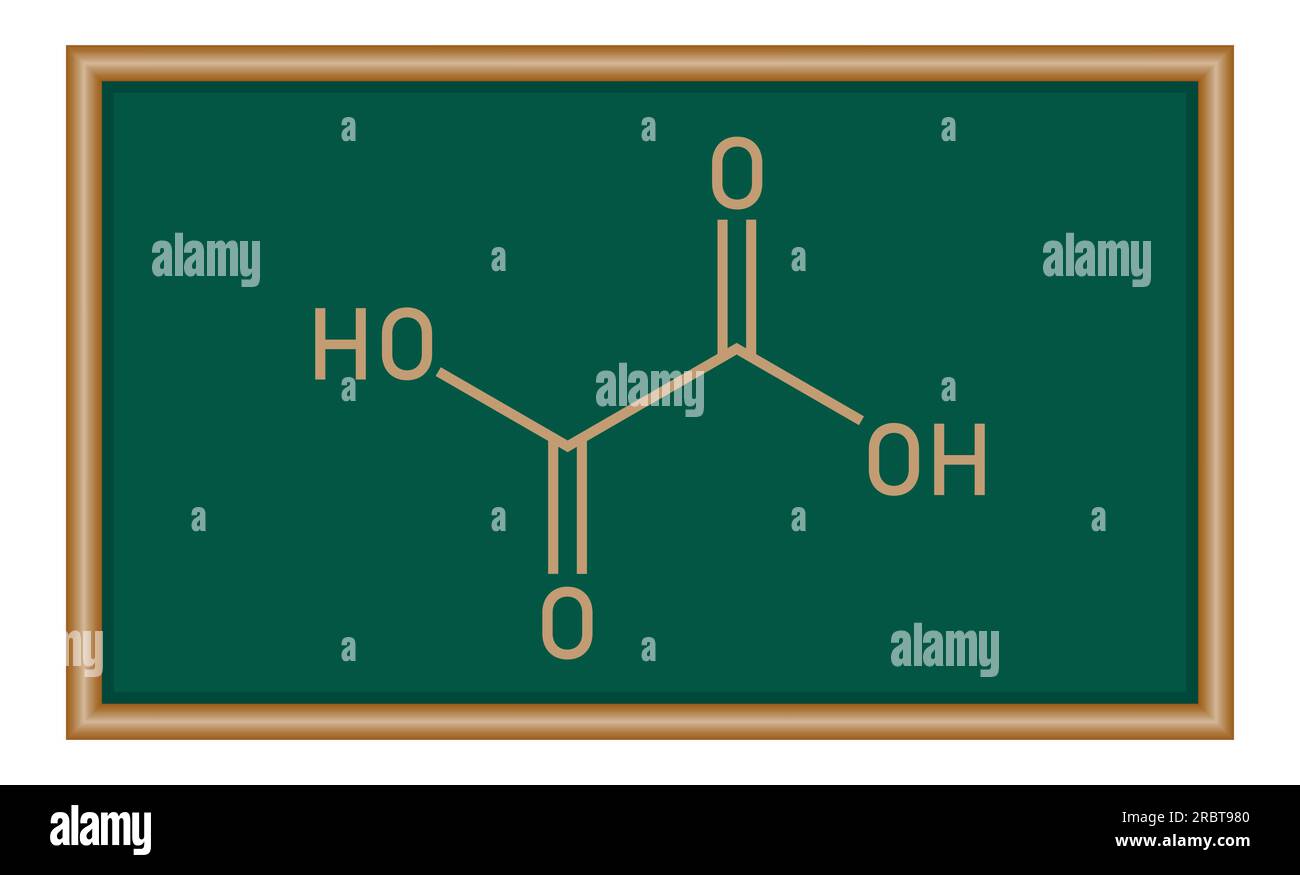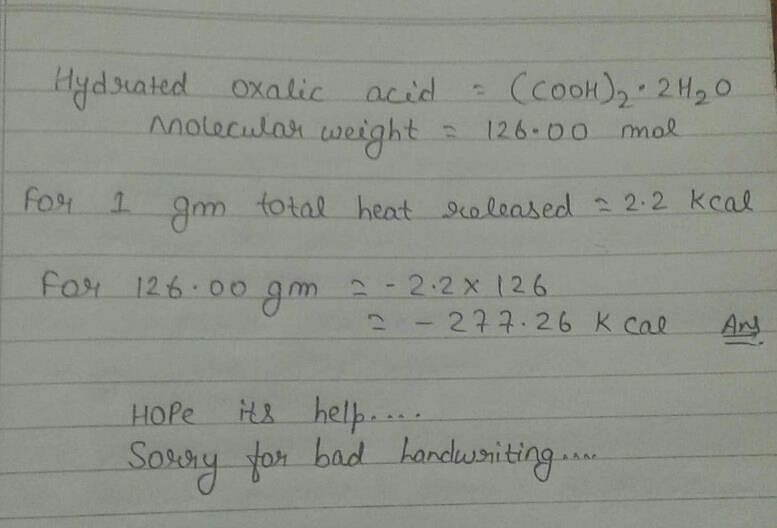
1 g hydrated oxalic acid upon combustion produces 2.2kcal of heat. It's enthalpy of combustion is -277. 2 kcal. Can someone explain how? - EduRev NEET Question
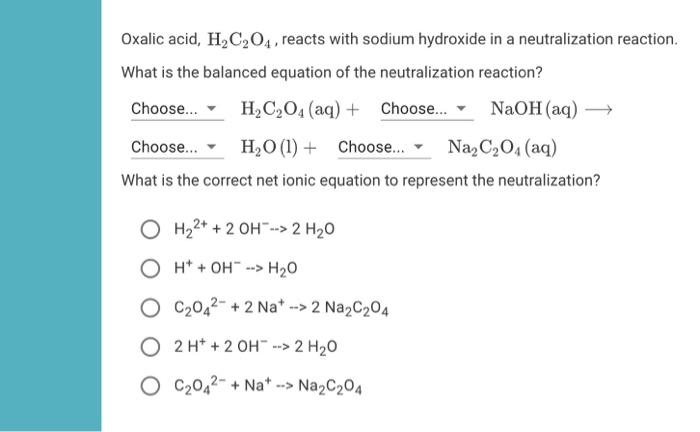
What mass of oxalic acid dihydate, H2C2O4•2H2O, is needed to make a 0.498 M solution of oxalic acid in a 250.0 mL volumetric flask? - Quora
Balance the following redox equation by half reaction method : H2C2O4(aq) + MnO4^Θ(aq) → CO2(g) + Mn^(2⊕)(aq)(acidic) - Sarthaks eConnect | Largest Online Education Community

diketahui persamaan reaksi H2C2O4 + H2O <-----> H3O + HC2O4 tentukan pasangan asam basa konjugasi - Brainly.co.id
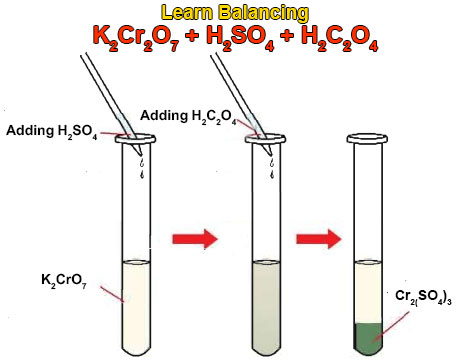
Balance KMnO4 + H2C2O4 + H2SO4 gives rise to K2SO4 + Mnso4 + CO2 + H2O using the alternate method of balancing

H2C2O4+H2O=C2O4+H3O balance the chemical equation @mydocumentary838. #hashtagvideo #youtube - YouTube