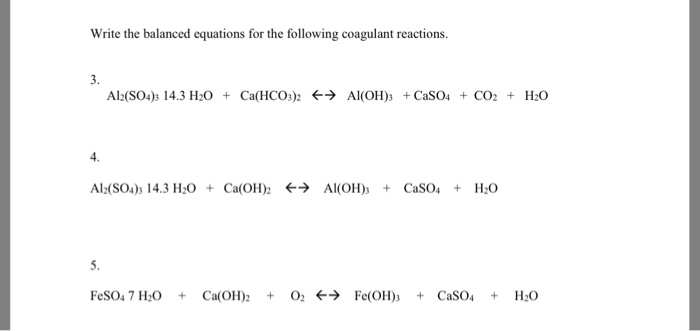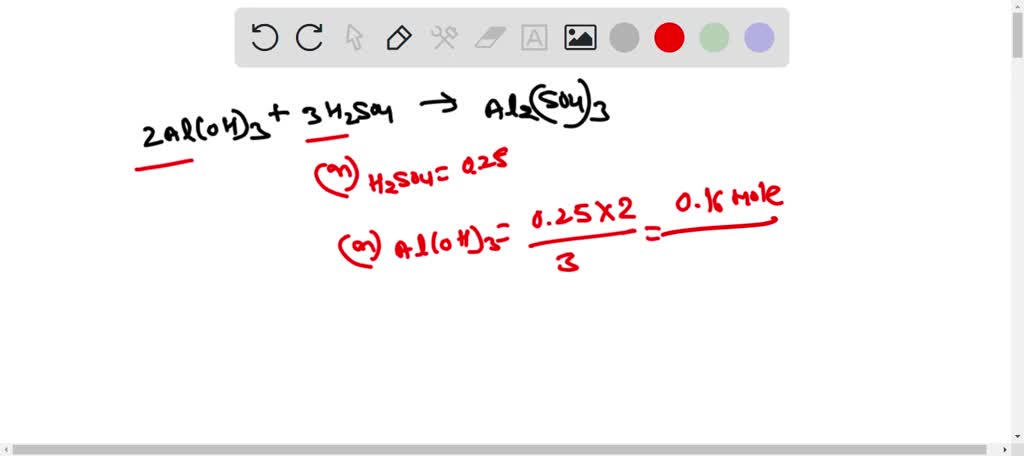
SOLVED: Use the following information to complete the table. Balanced equation: 2 Al(OH)3 + 3 H2SO4 → Al2(SO4)3 + 6 H2O Al(OH)3 H2SO4 Al2(SO4)3 H2O 88.003 g/mol 98.078 g/mol 342.15 g/mol 18.015

Consider the reaction of Al2O3 withH2SO4 to form Al2(SO4)3 and H2O. If 3.84 g AL2O3 is reacted with excess - brainly.com

Aluminium sulphate solution 0.2 % Al2(SO4)3 * 18 H2O for blue number determination Contents:, 48,91 €

Non-Ferric Aluminium Sulphate (Formula: Al2 (SO4) 3 X H2O (X 14-18) - China Nonferric, Iron Free | Made-in-China.com
:no_upscale()/3000.jpg)
19516.3000 - Aluminium sulfate solution, 40 % Al2(SO4)3 * 18 H2O/l, technical grade, 1 L | Analytics-Shop

Solved) - Al(OH)3(s) + H2SO4(aq) Al2(SO4)3(aq) + H2O(l) Express your answer... (1 Answer) | Transtutors

Phase Diagrams of (NH4)2SO4–Al2(SO4)3–H2O Ternary System: Effect of Sulfuric Acid and Its Application in Recovery of Aluminum from Coal Fly Ash | Journal of Chemical & Engineering Data


![Punjabi] Balance the chemical equation : Al(OH)3 + H2SO4 → Al2(SO4)3 Punjabi] Balance the chemical equation : Al(OH)3 + H2SO4 → Al2(SO4)3](https://static.doubtnut.com/ss/web-overlay-thumb/10301342.webp)





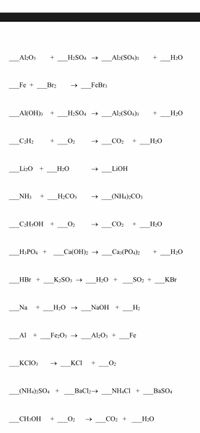


![Aluminium Sulfate Octahydrate [Al2(SO4)3.8H2O] Molecular Weight Calculation - Laboratory Notes Aluminium Sulfate Octahydrate [Al2(SO4)3.8H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/aluminium-sulfate-octahydrate-molecular-weight-calculation-300x178.jpg)